- Materials
-
- Flush Buffer (FB)
- Flush Tether (FLT)
- Loading Beads II (LBII)
- Sequencing Buffer II (SBII)
- Loading Solution (LS)
- Consumables
-
- 1.5 ml Eppendorf DNA LoBind tubes
- Equipment
-
- MinION device
- SpotON Flow Cell
- P1000 pipette and tips
- P100 pipette and tips
- P20 pipette and tips
-
Using the Loading Solution
We recommend using the Loading Beads II (LBII) for loading your library onto the flow cell for most sequencing experiments. However, if you have previously used water to load your library, you must use Loading Solution (LS) instead of water.
Note: some customers have noticed that viscous libraries can be loaded more easily when not using Loading Beads II. -
Thaw the Sequencing Buffer II (SBII), Loading Beads II (LBII) or Loading Solution (LS, if using), Flush Tether (FLT) and one tube of Flush Buffer (FB) at room temperature before mixing the reagents by vortexing and spin down at room temperature.
-
To prepare the flow cell priming mix, add 30 µl of thawed and mixed Flush Tether (FLT) directly to the tube of thawed and mixed Flush Buffer (FB), and mix by vortexing at room temperature.
-
Open the MinION device lid and slide the flow cell under the clip.
Press down firmly on the flow cell to ensure correct thermal and electrical contact.
-
Optional actionComplete a flow cell check to assess the number of pores available before loading the library.
This step can be omitted if the flow cell has been checked previously.
See the flow cell check instructions in the MinKNOW protocol for more information.
-
Slide the flow cell priming port cover clockwise to open the priming port.
-
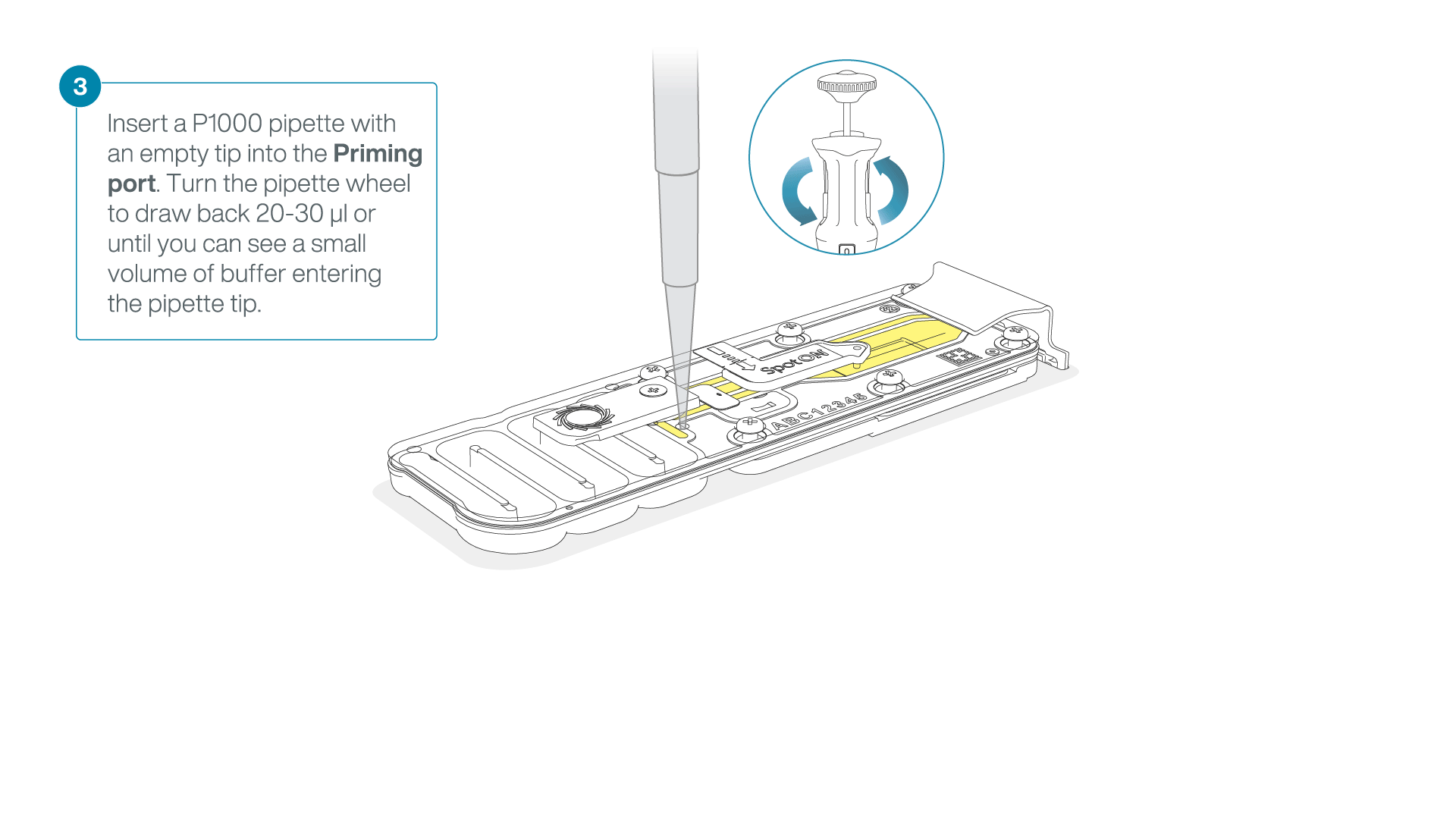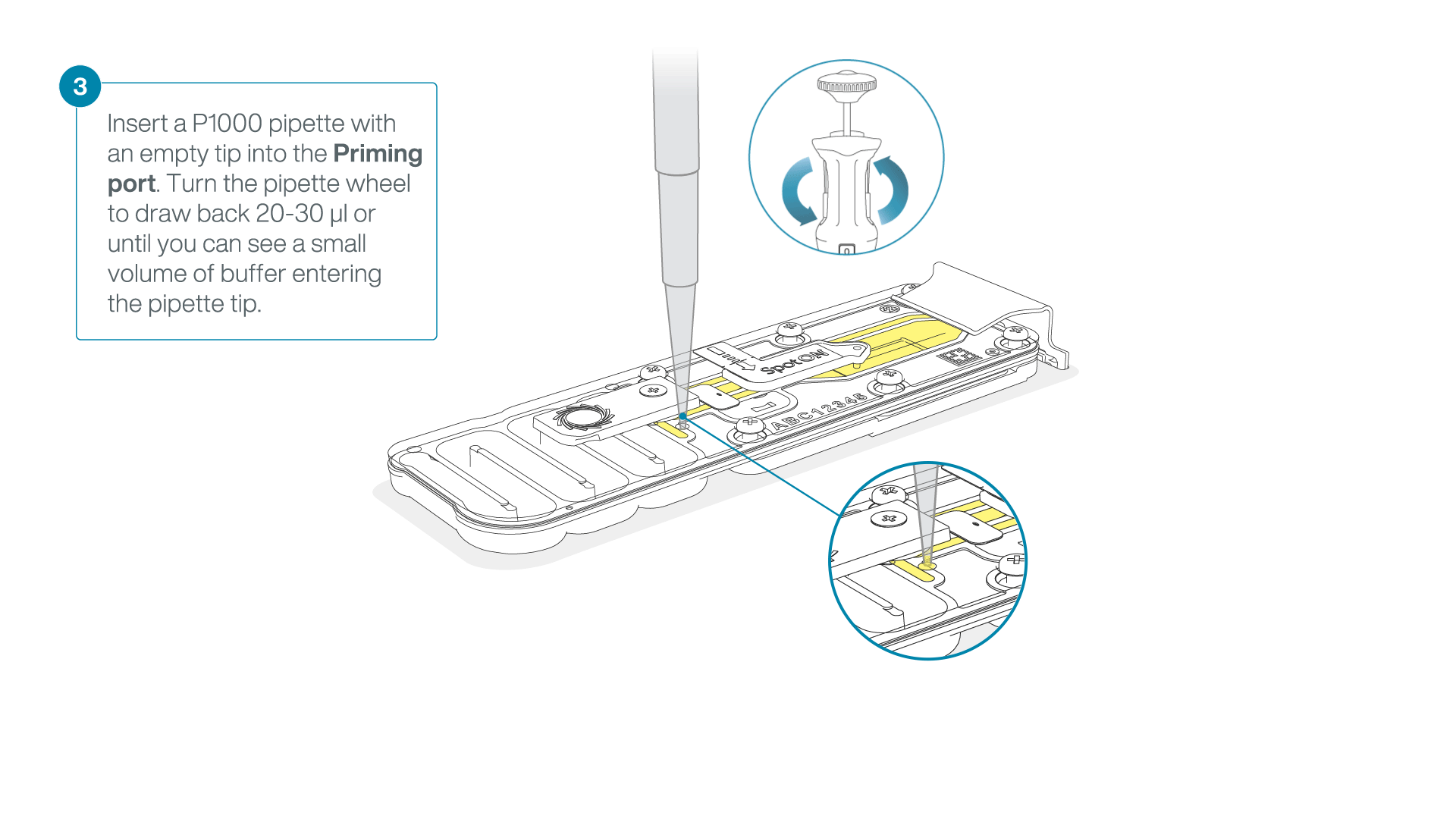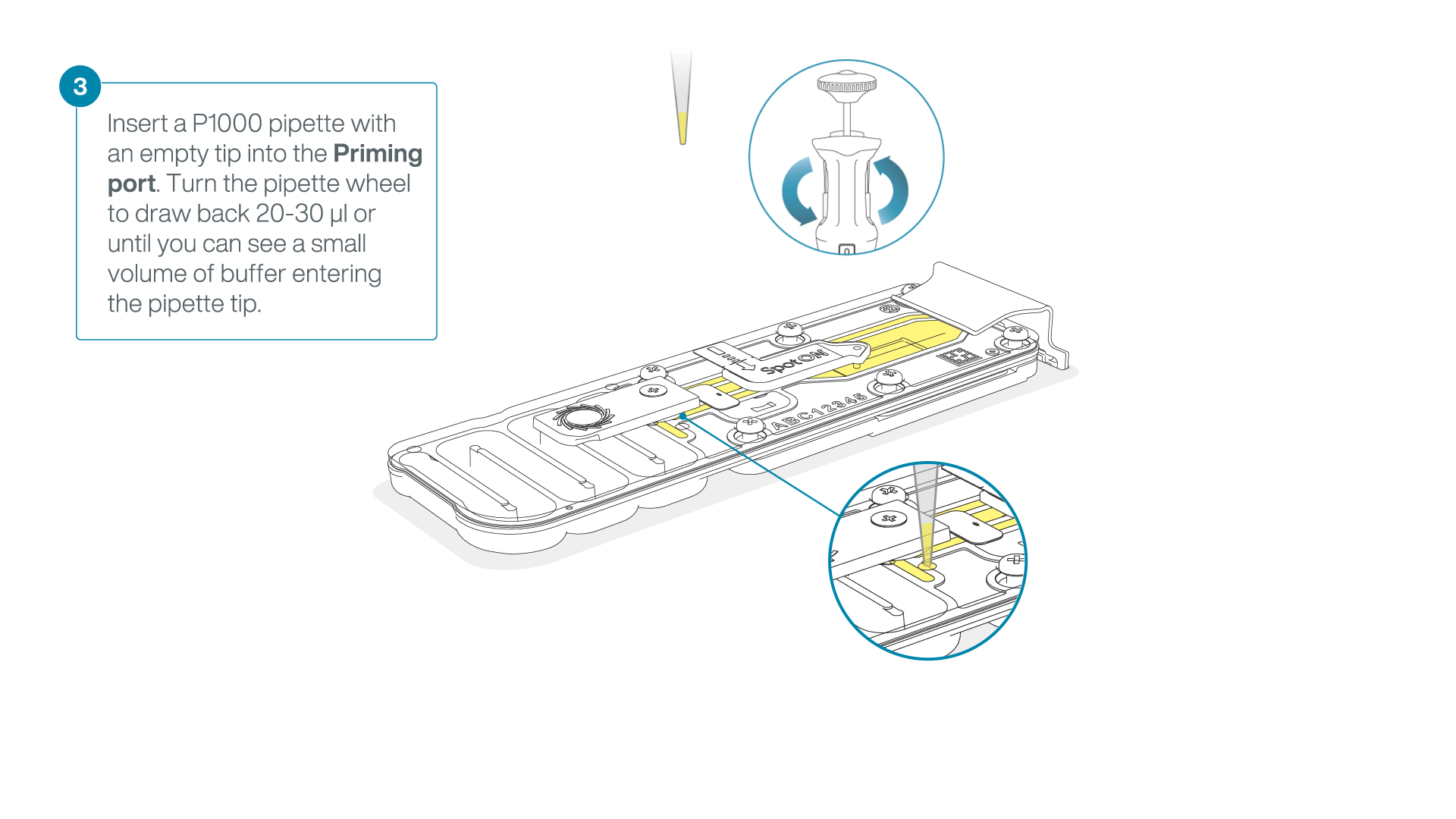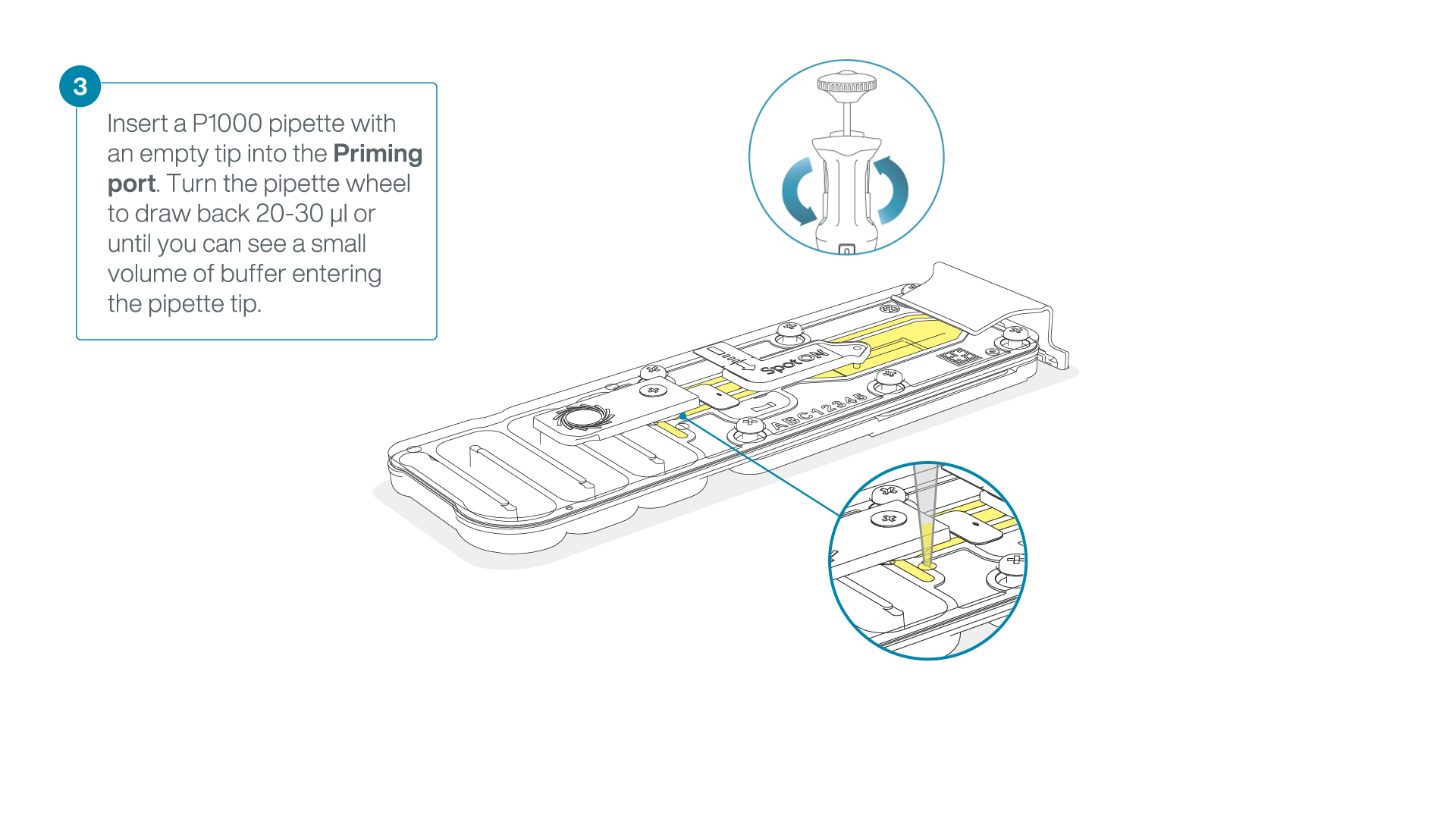
After opening the priming port, check for a small air bubble under the cover. Draw back a small volume to remove any bubbles:
- Set a P1000 pipette to 200 µl
- Insert the tip into the priming port
- Turn the wheel until the dial shows 220-230 µl, to draw back 20-30 µl, or until you can see a small volume of buffer entering the pipette tip
Note: Visually check that there is continuous buffer from the priming port across the sensor array.
-
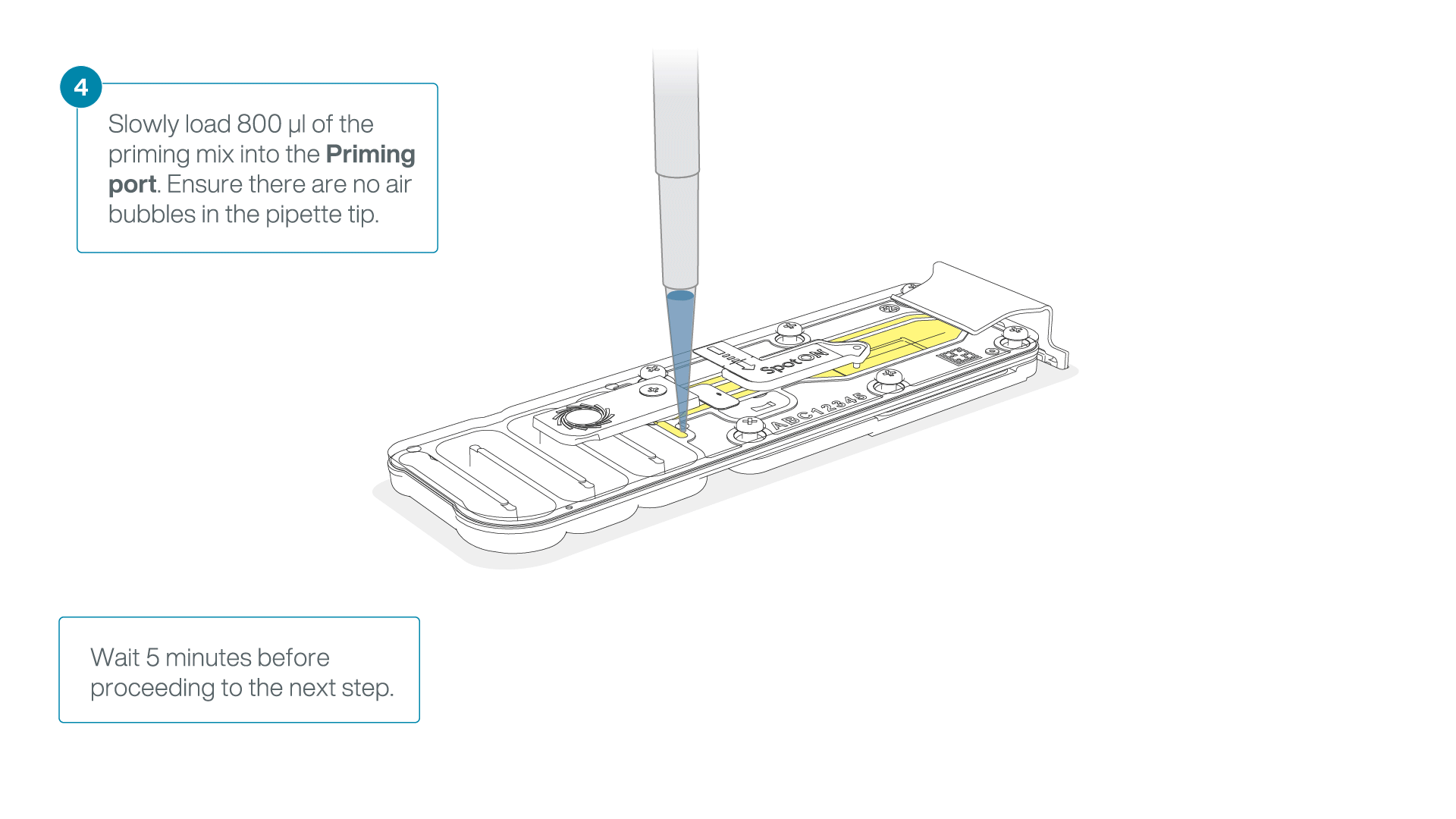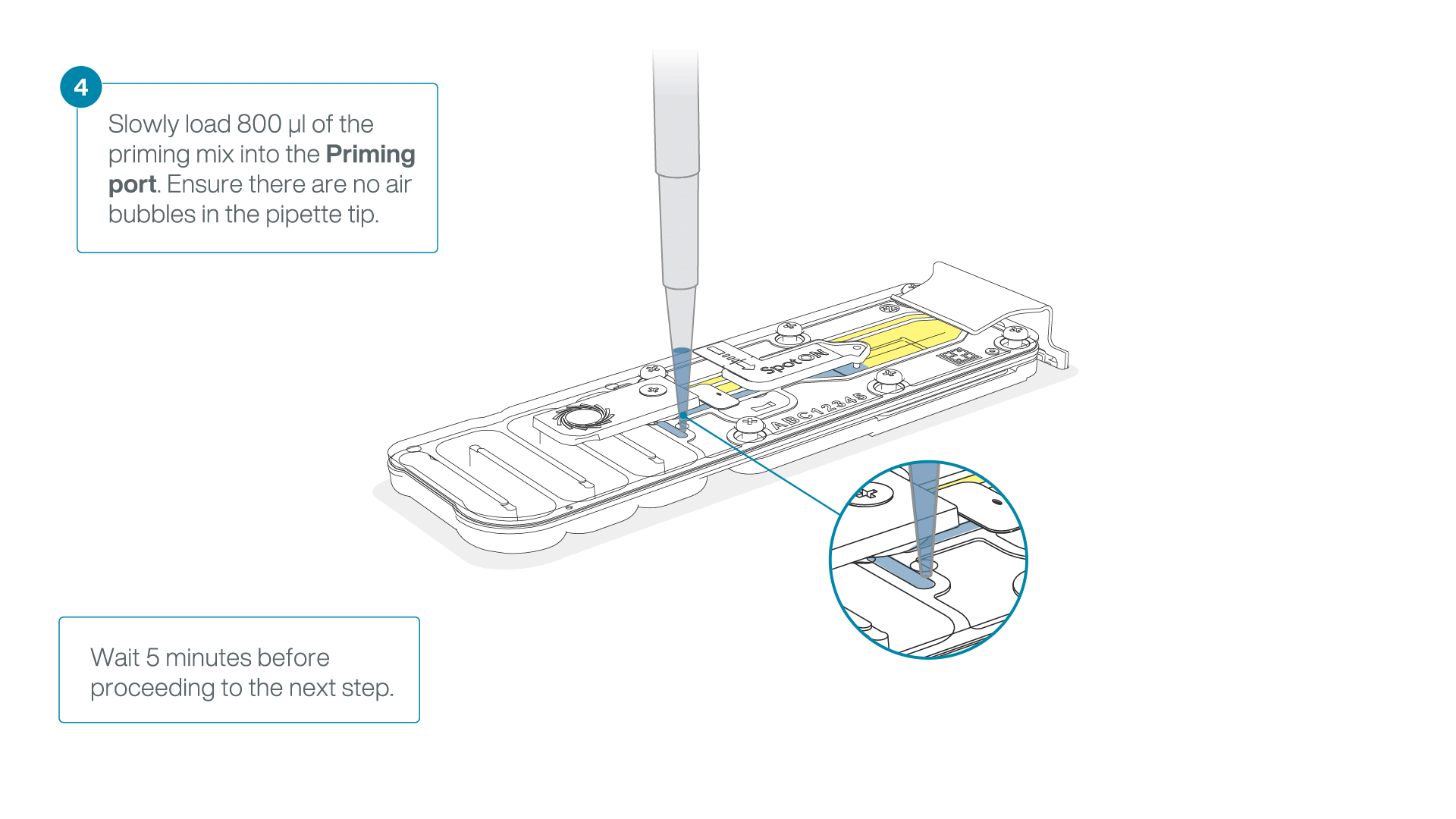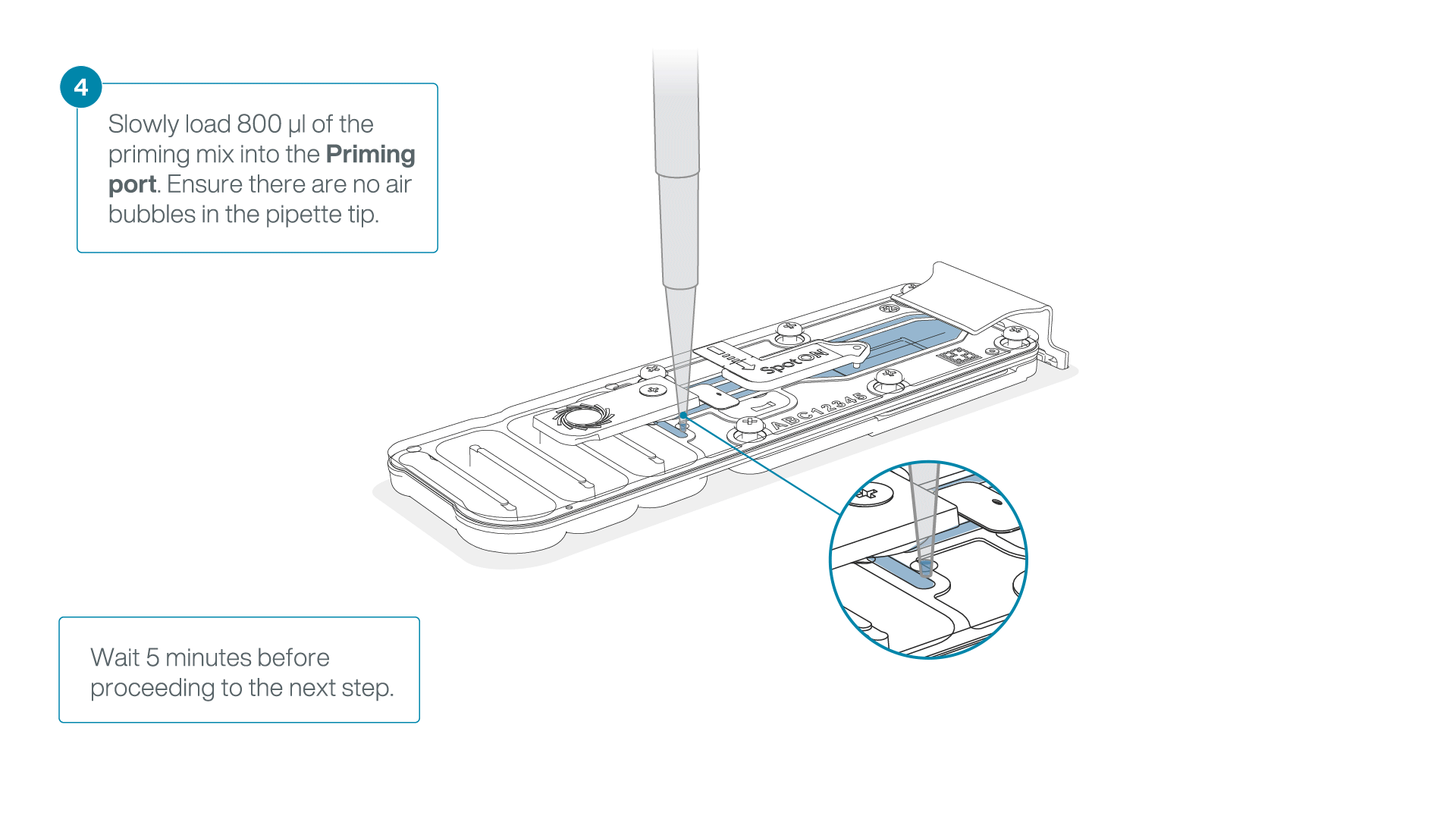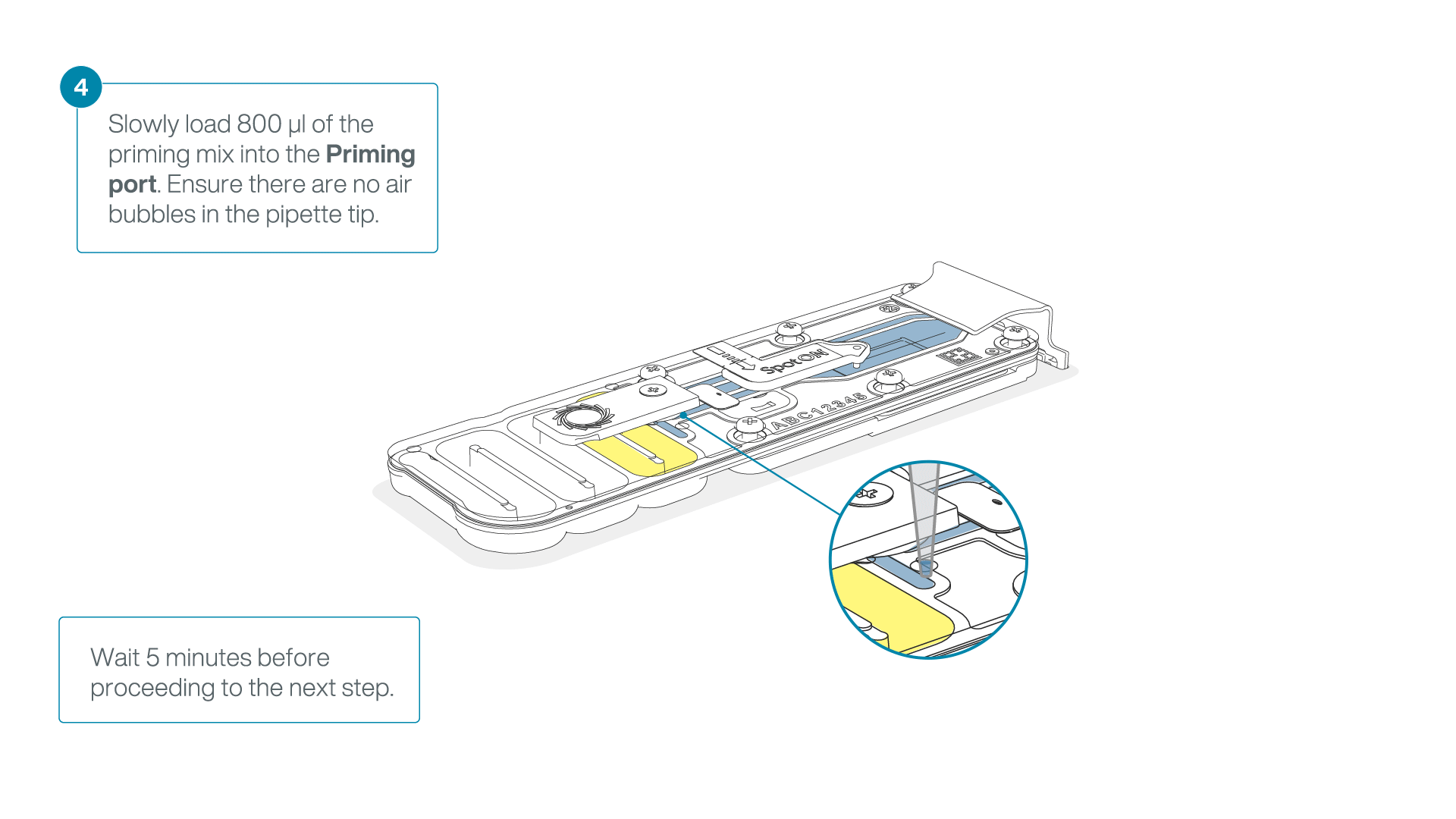
Load 800 µl of the priming mix into the flow cell via the priming port, avoiding the introduction of air bubbles. Wait for five minutes. During this time, prepare the library for loading by following the steps below.
-
Thoroughly mix the contents of the Loading Beads II (LBII) by pipetting.
-
In a new tube, prepare the library for loading as follows:
Reagent Volume per flow cell Sequencing Buffer II (SBII) 37.5 µl Loading Beads II (LBII) mixed immediately before use, or Loading Solution (LS), if using 25.5 µl DNA library 12 µl Total 75 µl Note: Load the library onto the flow cell immediately after adding the Sequencing Buffer II (SBII) and Loading Beads II (LBII).
-
Complete the flow cell priming:
- Gently lift the SpotON sample port cover to make the SpotON sample port accessible.
- Load 200 µl of the priming mix into the flow cell priming port (not the SpotON sample port), avoiding the introduction of air bubbles.
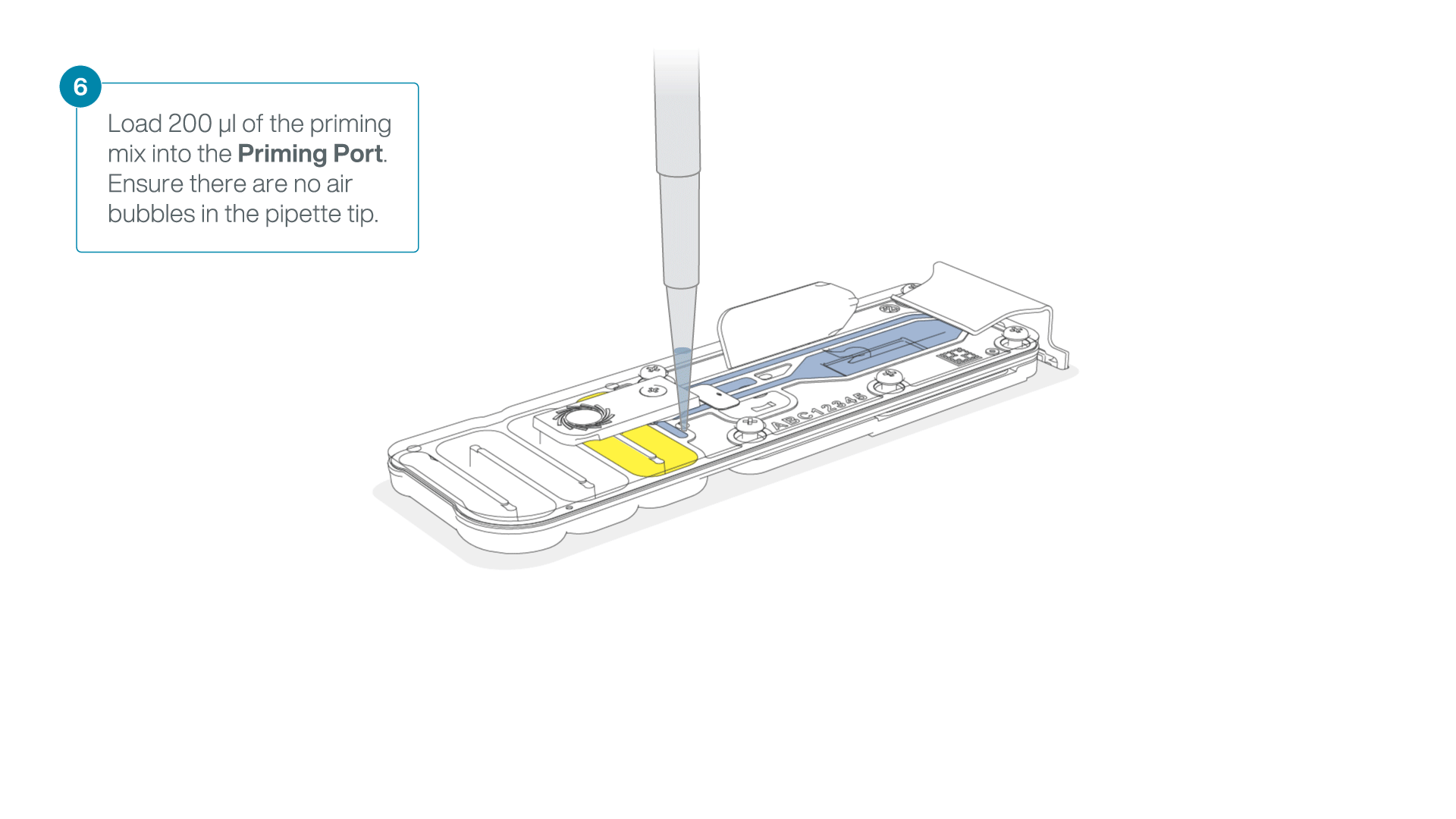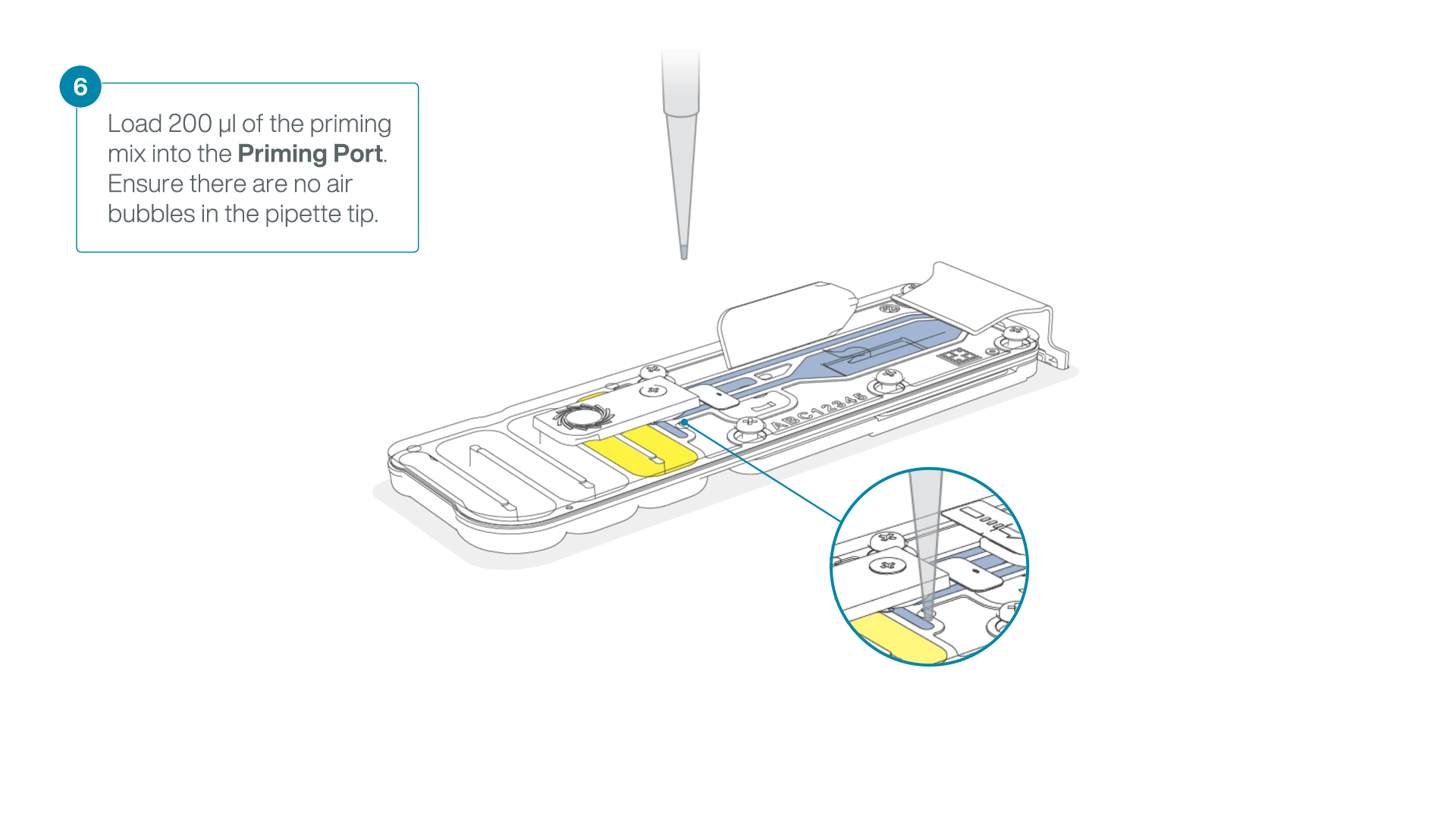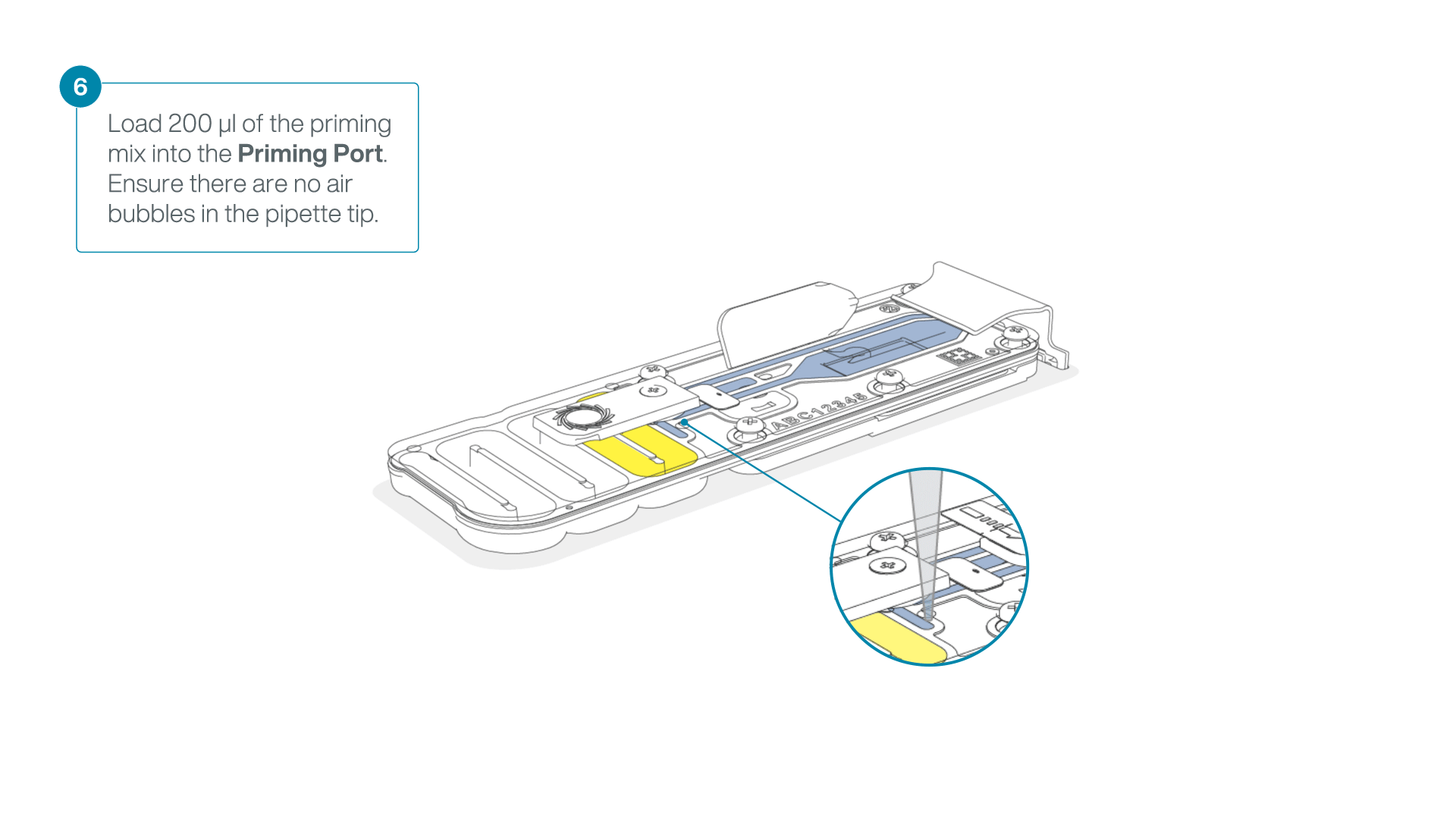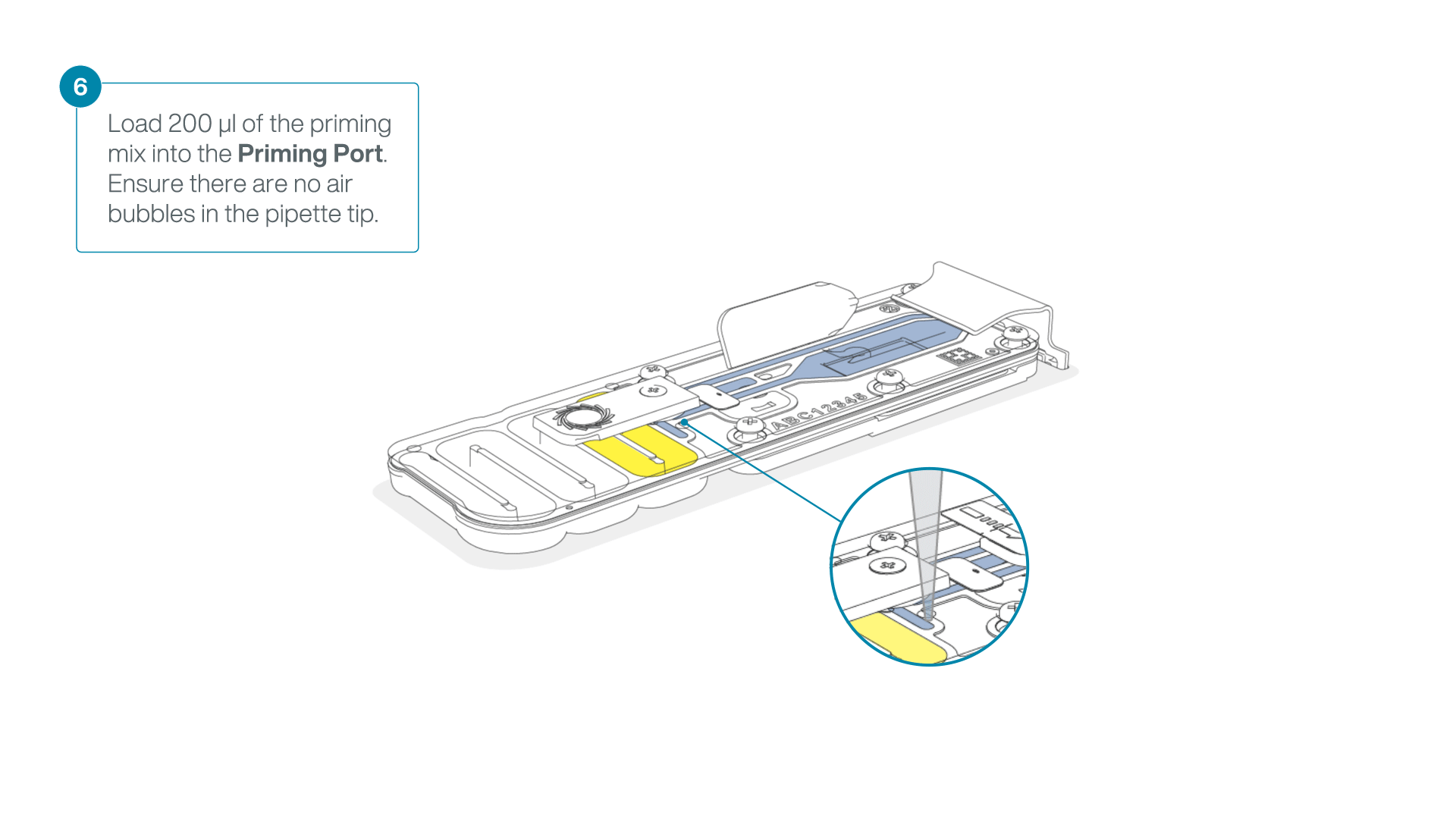
-
Mix the prepared library gently by pipetting up and down just prior to loading.
-
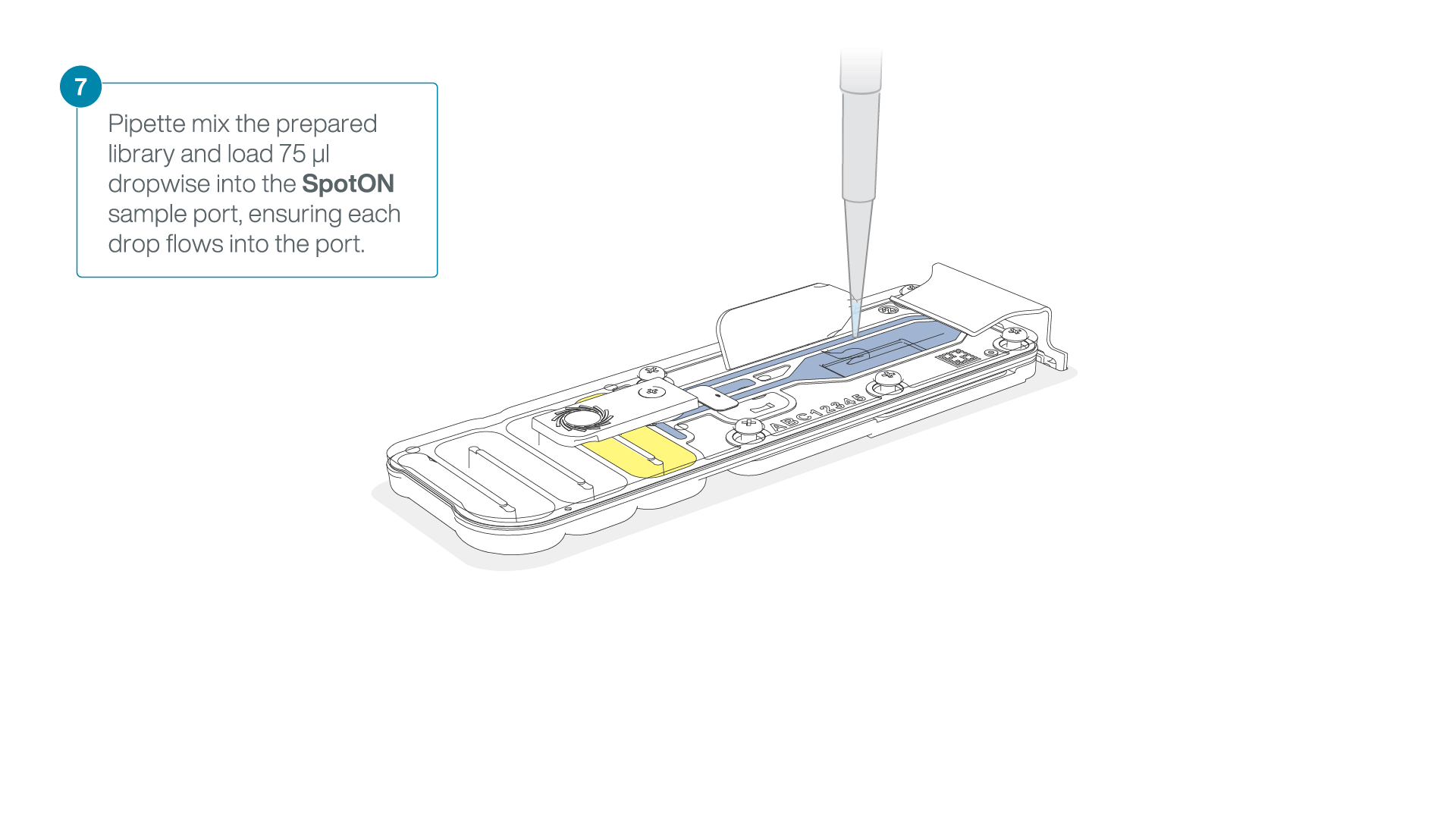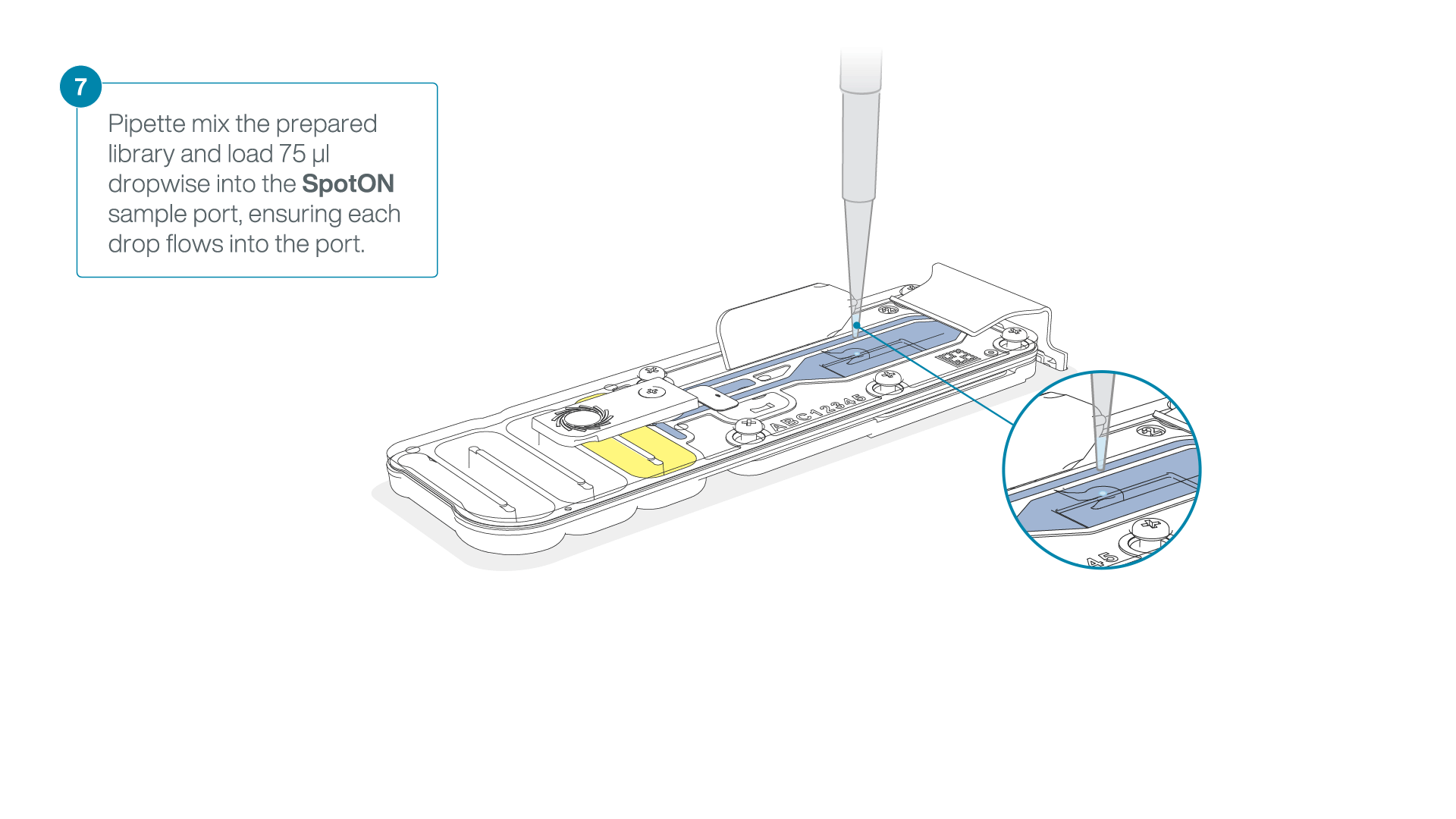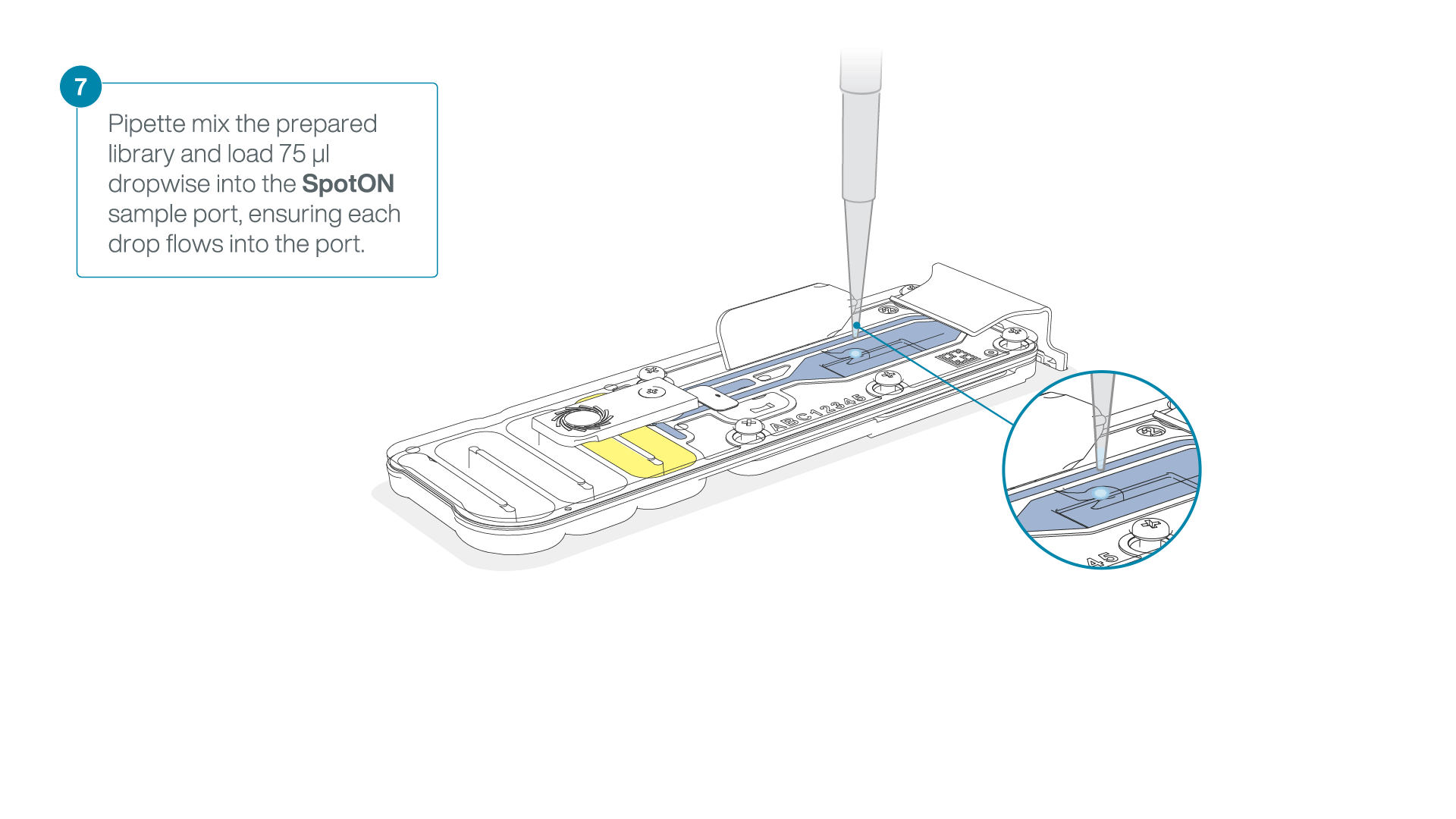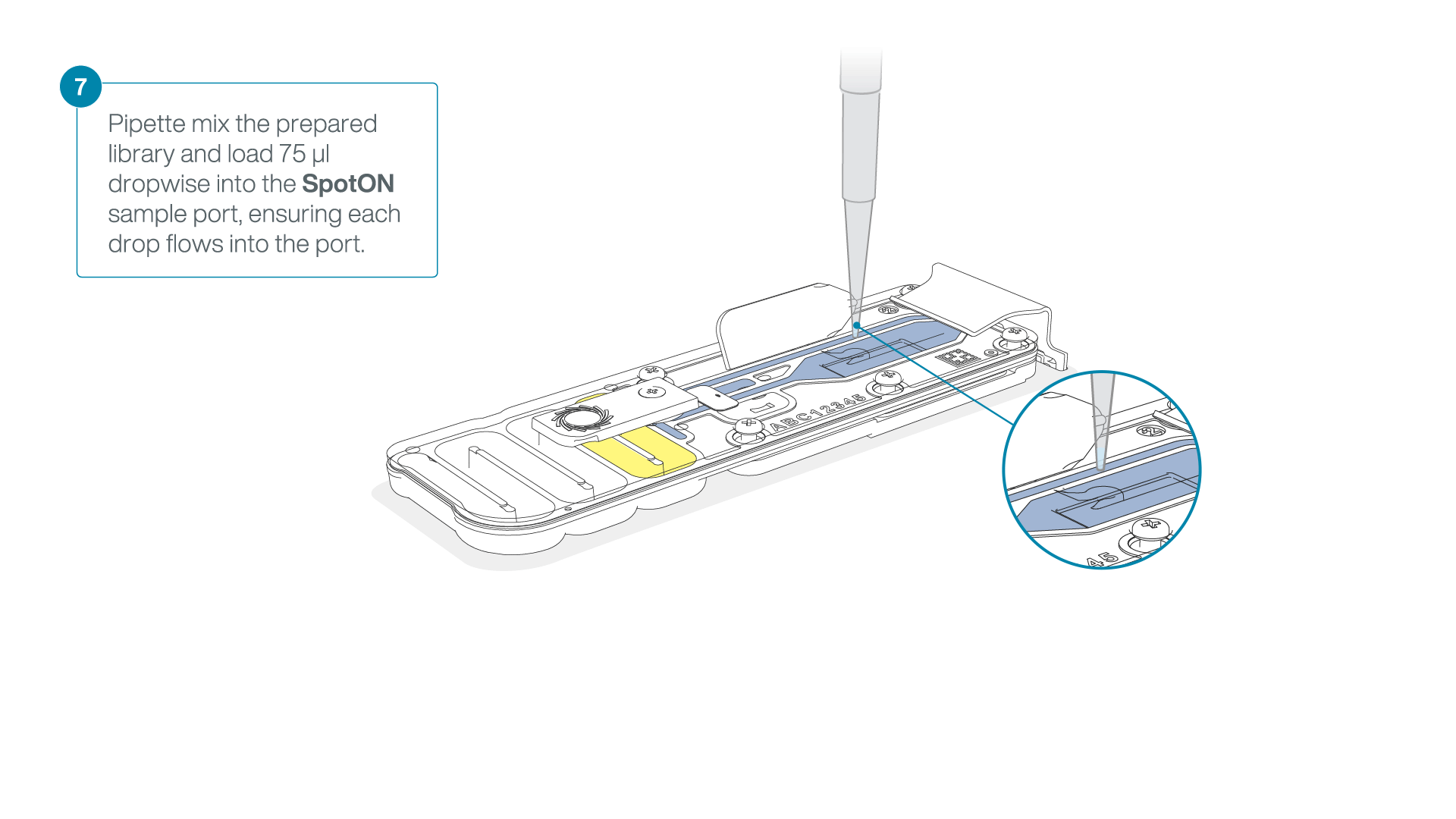
Add 75 μl of the prepared library to the flow cell via the SpotON sample port in a dropwise fashion. Ensure each drop flows into the port before adding the next.
-
Gently replace the SpotON sample port cover, making sure the bung enters the SpotON port, close the priming port and replace the MinION device lid.



